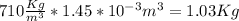Answer: One canister contains 1.03 Kg of fuel,
Step-by-step explanation:
The density is defined as the relation between the mass and the volume
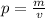
First of all you need to have the same units for volume and density, so:
For the volume,
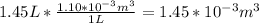
For the density,
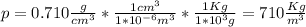
From the density equation we have
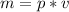 , so:
, so: