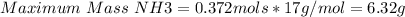Answer:
Maximum amount of ammonia that can be formed is 6.32 g
Step-by-step explanation:
The balanced equation is:
N2 + 3H2\rightarrow 2NH3
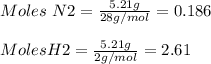
N2 is the limiting reactant
Based on the reaction stoichiometry:
1 mole N2 produced 2 moles NH3
therefore, 0.186 mol N2 will yield: 2(0.186) = 0.372 moles NH3
Molar mass NH3 = 17 g/mol