Answer: 708.2 grams
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
Given : 38 g of
 is dissolved in 100 g of solution.
is dissolved in 100 g of solution.
Density of solution = 1.285 g/ml
Volume of solution =
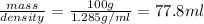
Thus if 77.8 ml of
 contains = 38 g of
contains = 38 g of

1.45L= 1450 ml of
 contains =
contains =
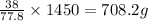 of
of

Therefore, the mass of the sulfuric acid in a car battery is 708.2 g