Answer:
which orbital is higher in energy?
The 3s orbital is higher in energy.
Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron?
It will require less energy to remove a 3s electron from the chlorine atom.
Explanation:
which orbital is higher in energy?
The energy will be higher for the case of the 3s orbital since it is closer to the nucleus of chlorine atom, that means that the electrons in this orbital will be submitted to a bigger electric force.
The protons in the nucleus of the chlorine atom have a positive charge and the electrons have a negative charge, so they will be attracted to each other (equal charges repeal, opposite charges attract). It is known that the electric force will decrease with the square of the distance between two charges (inversely proportional) as is establish in Coulomb’s law:
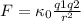 (1)
(1)
Where q1 and q2 are the charges,
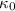 is the proportionality constant and r is the distance between the two charges.
is the proportionality constant and r is the distance between the two charges.
Since the 3s orbital is closer to the nucleus, the electrons in there will be more attracted to the nucleus according to Coulomb’s law.
The general idea is:
Closer orbitals will have higher energy because they are closer to the nucleus of its atom and the electric force will be stronger for the particles of different charges inside of it.
Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron?
Electrons are in shells (orbitals) of probabilities of their position around the atom. To understand how they are distributed inside this orbitals it is necessary to the define what is the “electronic configuration of an atom”.
Electronic configuration: Shows how electrons from an atom are distributed in different orbitals, in which each orbital has a certain amount of electrons that can hold.
The notation of how these orbitals are arranged to be filled by the electrons is schematized below:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p
In which 1s correspond to the orbital closest to the nucleus, and 7p the outermost.
So as it was already explained, orbitals that are closer to the nucleus have a greater energy associated. For the case of 3s electron it will require less energy to be removed than the 2p electron since the 3s orbital is farther than the 2p orbital to the nucleus.