Answer:
The mass in kilograms is 64.52
Step-by-step explanation:
We know that density of a substance is an intensive property that can be calculated with following formula:
![[tex]density= (mass)/(volume)](https://img.qammunity.org/2020/formulas/physics/high-school/nxk42mzhqpaqsxgvd138w79woh9qx00ejq.png) [/tex]
[/tex]
Using the formula and using simple 3 rule, we can calculate the mass in the room.
First we calculate the volume of the room:
![[tex]Volume=lenght*height*with](https://img.qammunity.org/2020/formulas/physics/high-school/roevcfydct3srgqikm5748vo4flbsg0t09.png) \\
\\
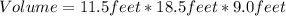 \\
\\
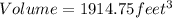 [/tex]
[/tex]
As density is given in grams/liters, we need to change unit measure feet3 to liters:
![[tex]1 ft^(3) ---------28.32L\\ 1914.75ft^(3) -----------------------54225.72 L](https://img.qammunity.org/2020/formulas/physics/high-school/k6tzs0qwtwmm6zmrxhfb1e45r8sqh1pirn.png) [/tex]
[/tex]
Now we can use density formula isolating the mass, to get the value needed:
![[tex]mass=density*volume](https://img.qammunity.org/2020/formulas/physics/high-school/fjs6mylfxqlejwkpkqfcatfj19wgwwv7cu.png) \\[/tex]
\\[/tex]
Following step is to replace density and volume with the data that we have:
![[tex]mass=1.19g/L*54225.72L\\ mass=64528,60g](https://img.qammunity.org/2020/formulas/physics/high-school/blbbhvxp6kjdbvhqn5jizv2ri6k4hjahkc.png) [/tex]
[/tex]
We know that 1000 g is 1Kg, so to obtain the value in Kg we need to divide the result.
![[tex]mass=64.52Kg](https://img.qammunity.org/2020/formulas/physics/high-school/z27qi8i3z4wj7gwl0045wcui2pyi4o9rvm.png) [/tex]
[/tex]