Answer:
The Temperature of the Hot reservoir should be increased by 359.86°C
Step-by-step explanation:
A Carnot engine is a reversible engine, that means that it can work both as a heat engine and as a refrigerator, it can both use heat from a hot reservoir (part of which later must end up in a cold reservoir) to produce work, or use work to move heat from a cod to a hot reservoir.
The efficiency of a carnot engine working as a heat engine is given by:
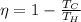
were
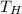 and
and
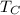 are the Absolute temperatures of the hot and cold reservoir respectively.
are the Absolute temperatures of the hot and cold reservoir respectively.
To get the absolute temperature in K from the relative temperature in °C, we must add 273.15 K (the absolute temperature of the freezing point of water at atmospheric pressure)
Thus:
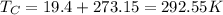
Now, if we know the Temperature of the cold reservoir, and the engine's efficiency, we can use that information to get
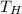 as follows:
as follows:
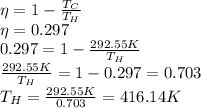
Now, that is the Temperature of the hot reservoir to begin with, but if we want a higher efficency, we need to increase
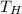 until
until
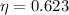 .
.
To find out what value
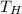 has to reach, we repeat the same calculation as before, except now
has to reach, we repeat the same calculation as before, except now
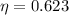
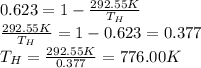
So
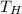 has increased from
has increased from
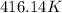 to
to
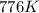 , that means an increase in temperature of :
, that means an increase in temperature of :
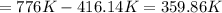
This increment of 359.86K is also an increment of 359.86°C, because increments are relative measures of temperature.