Step-by-step explanation:
Nuclear reactions are defined as the reactions in which there occurs change in the nucleus of an atom. As neither electrons or protons are not involved in a nuclear reaction.
For example,
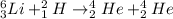
According to law of conservation of mass, mass can neither be created nor it can be destroyed but it can be converted into one form to another.
Hence, in nuclear reactions mass remains conserved.
Also, there is no involvement of electrons and protons. So, there occurs no change in charge of the atoms. Therefore, charge also remains conserved in a nuclear reaction.
Thus, we can conclude that all types of nuclear reactions have in common that all processes conserve total mass number and total charge. Whereas they differ in specific particle consumed and produced.