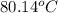Answer: The final temperature of the solution is
Step-by-step explanation:
The amount of heat released by coffee will be absorbed by aluminium spoon.
Thus,
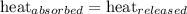
To calculate the amount of heat released or absorbed, we use the equation:
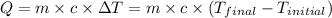
Also,
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2020/formulas/chemistry/college/bm2kxludvecqgwsul6e5upktie71evfnq2.png) ..........(1)
..........(1)
where,
q = heat absorbed or released
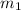 = mass of aluminium = 39 g
= mass of aluminium = 39 g
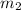 = mass of coffee = 166 g
= mass of coffee = 166 g
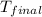 = final temperature = ?
= final temperature = ?
 = temperature of aluminium =
= temperature of aluminium =
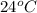
 = temperature of coffee =
= temperature of coffee =
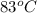
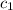 = specific heat of aluminium =
= specific heat of aluminium =
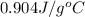
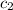 = specific heat of coffee=
= specific heat of coffee=
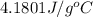
Putting all the values in equation 1, we get:
![39* 0.904* (T_(final)-24)=-[166* 4.1801* (T_(final)-83)]](https://img.qammunity.org/2020/formulas/physics/college/f8mxvec9swf1uvjej07fct3507xsxgxgin.png)
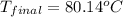
Hence, the final temperature of the solution is