Step-by-step explanation:
The given data is as follows.
Partition coefficient, K = 6.2
Volume of phase 1,
 = 74.0 mL
= 74.0 mL
Volume of phase 2,
 = 17.0 mL
= 17.0 mL
So, after one extraction fraction of solute remaining is given as follows.
q =
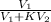
After 3 times extraction, fraction of S remaining is as follows.
q =
![[(V_(1))/(V_(1) + KV_(2))]^(3)](https://img.qammunity.org/2020/formulas/chemistry/college/fof73d8iaz2fruey20dheoaopfhh69admc.png)
Putting the given values into the above formula as follows.
q =
![[(V_(1))/(V_(1) + KV_(2))]^(3)](https://img.qammunity.org/2020/formulas/chemistry/college/fof73d8iaz2fruey20dheoaopfhh69admc.png)
=
![[(74.0 ml)/(74.0 ml + 6.2 * 17.0 ml)]^(3)](https://img.qammunity.org/2020/formulas/chemistry/college/3be589yvjv6g3cz39n6rjgiren8927mbvr.png)
=
![[(74.0 ml)/(179.4 ml)]^(3)](https://img.qammunity.org/2020/formulas/chemistry/college/e73qsh49nh59xka0q2i2wiouz1eqtsm2fo.png)
= 0.0699
Thus, we can conclude that the fraction of S remaining in the aqueous phase is 0.0699.