Answer: 2. cannot be an element.
Step-by-step explanation:
Element is a pure substance which is composed of atoms of similar elements.It can not be decomposed into simpler constituents using chemical reactions.Example: Copper
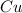
Compound is a pure substance which is made from atoms of different elements combined together in a fixed ratio by mass.It can be decomposed into simpler constituents using chemical reactions. Example: methane

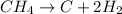
Thus as it can be decomposed into its constituent elements, it is a compound.
Mixture is a substance which has two or more components which do not combine chemically and do not have any fixed ratio in which they are present.
Thus methane cannot be an element.