Answer:
The molar solubility of NiS is 7.7 * 10⁻⁷ M
Step-by-step explanation:
To answer this question, we need to keep in mind two equilibriums.
First, we have the solubilization of NiS:
NiS ⇄ Ni²⁺ + S²⁻ ksp= 3.0 * 10⁻²¹ (we know this from standard tables)
Second, we have the formation of the complex:
Ni²⁺ + 6NH₃ ⇄ [Ni(NH₃)₆]²⁺ kf=2.0 * 10⁻⁸
Combine the two equilibriums and we have
NiS + 6NH₃ ⇄ [Ni(NH₃)₆]²⁺ + S²⁻ K= ksp * kf =6.0* 10⁻¹³=
![([S^(2-)][Ni(NH3)6^(+2)])/([NH3]^(6))](https://img.qammunity.org/2020/formulas/chemistry/college/vbmyit2ewa422qnkmwabnu75yjxejhr1nb.png)
The molar solubility s is equal to both [Ni(NH₃)₆²⁺] and [S²]
At equilibrium, [NH₃]= 3,1 M - 6s
Thus, if we replace those terms in the formula for K, we're left with:
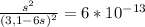
Using an approximation we can ignore the denominator and we have