Answer: The isotopic abundance of Cl- 37 is 0.2424.
Step-by-step explanation:
Mass of isotope - 35 = 34.96885 amu
% abundance of isotope -35= x% =
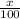
Mass of isotope - 37 = 36.9659 amu.
% abundance of isotope-37 = (100-x)% =
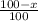
Formula used for average atomic mass of an element :
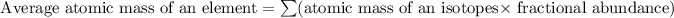
![35.453=\sum[(34.96885 * (x)/(100))+(36.9659 * (100-x)/(100)]]](https://img.qammunity.org/2020/formulas/chemistry/high-school/tbkgew6tn9dgby9t4ncvcgob1wbnajw30z.png)
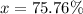
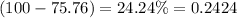
Therefore, the isotopic abundance of Cl- 37 is 0.2424.