Answer:
The answer is D, only certain energies are allowed for the electron in a hydrogen atom.
Step-by-step explanation:
We know that light quanta, also called photons have energy:
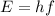 Where h is Planck's constant and f is the frequency of the photon. The frequency of a photon tells us the colour of the light, different colours have different frequencies, and white light contains photons of all frequencies in the visible range. But the light emitted by Hydrogen in a low pressure tube that is excited by an electric current contains only photons from a few frequencies.
Where h is Planck's constant and f is the frequency of the photon. The frequency of a photon tells us the colour of the light, different colours have different frequencies, and white light contains photons of all frequencies in the visible range. But the light emitted by Hydrogen in a low pressure tube that is excited by an electric current contains only photons from a few frequencies.
Why is that? Quantum mechanics explains us that it is because an electron that is bound to a hydrogen atom can take many configurations, of different energies, the configuration for which the electron is as close as possible to the nucleus (in this case a single proton) is called the ground state, and the other higher energy states are called excited states. if an electron is in an excited state, it will tend to "jump" down into the ground state, emitting light in the process. That light is the light we see, and its spectrum is discrete because only a few energies are allowed for the electron in a hydrogen atom.
That is why D is true.
Why are the other questions wrong?
A: Wrong because hydrogen can only form
 molecules, and even so, the great energy levels in the tube destroy the molecule leaving only single free H atoms.
molecules, and even so, the great energy levels in the tube destroy the molecule leaving only single free H atoms.
B: The prism's only job is to separate light into different frequencies so that we can see the different colours, it won't block any lines.
C: Wrong because hydrogen atoms only have a single electron.
E: wrong because if the spectrum was continuous we wouldn't see single lines, but a continuous range of colours, like in white light.