Answer:
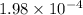 mg
mg
Explanation:-
According to avogadro's law, 1 mole of every substance occupies 22.4 Liters at STP , contains avogadro's number
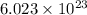 of particles and weigh equal to the molecular mass of the substance.
of particles and weigh equal to the molecular mass of the substance.
To calculate the moles, we use the equation:
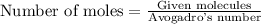
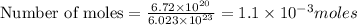
Now 1 mole of
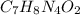 molecule weigh = 180g
molecule weigh = 180g
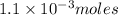 of
of
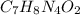 molecule weigh =
molecule weigh =
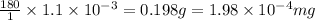 (1g=1000mg)
(1g=1000mg)
Thus
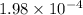 mg of theobromine are present in the sample.
mg of theobromine are present in the sample.