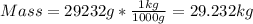Answer: 29.232 kg
Explanation: First, we must calculate the volume of the room. From the dimensions given in the exercise, we can assume that the room is box-shaped so its volume will be:
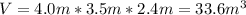
Since the density of nitrogen in air is given in grams over liter, we must change the volume units from cubic meters to litres.
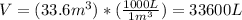
Now we just have to multiply the volume of the room by the density of nitrogen:
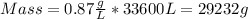
To report this mass in kilograms, unit conversion is applied: