Answer:
electrostatic force = 1.08 ×
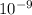 N
N
and if sodium ion replace by Li+ and chloride by Br− then there will be no change in electrostatic force
magnitude of electrostatic force will be same
Step-by-step explanation:
given data
separation d = 0.46 nm = 4.6 ×
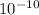 m
m
to find out
electrostatic force between Na+ and Cl− ion
and if sodium ion replace by Li+ and chloride by Br−
solution
we know electrostatic force formula that is
electrostatic force = K ×Q1×Q2 / d² .........................1
here k is coulombs constant that is = 9 ×
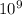 and Q1 and Q2 is
and Q1 and Q2 is
charge that is Q1 = + 1.6 ×
 C
C
and Q2 = - 1.6 ×
 C
C
so put all these value in equation 1
electrostatic force = K ×Q1×Q2 / d²
electrostatic force = 9 ×
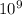 ×1.6 ×
×1.6 ×
 × -1.6 ×
× -1.6 ×
 / (4.6 ×
/ (4.6 ×
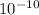 )²
)²
so electrostatic force = 1.08 ×
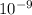 N
N
and if sodium ion replace by Li+ and chloride by Br− then there will be no change in electrostatic force
so magnitude of electrostatic force will be same