Answer:
density is
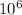 Mg/µL
Mg/µL
Step-by-step explanation:
given data
density of nuclear =
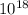 kg/m³
kg/m³
1 ml = 1 cm³
to find out
density of nuclear matter in Mg/µL
solution
we know here
1 Mg = 1000 kg
so
1 m³ is equal to
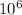 cm³
cm³
and here 1 cm³ is equal to 1 mL
so we can say 1 mL is equal to 10³ µL
so by these we can convert density
density =
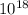 kg/m³
kg/m³
density =
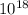 kg/m³ ×
kg/m³ ×
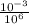 Mg/µL
Mg/µL
density =
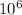 Mg/µL
Mg/µL