Answer: 84400 Joules
Step-by-step explanation:
Molar enthalpy of fusion for water = 6.008 kJ/mol
Molar heat of fusion is the energy required to convert 1 mole of solid to 1 mole of liquid.

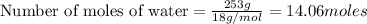
1 mole of liquid water on freezing releases energy = 6.008 kJ
Thus 14.06 moles of liquid water on freezing releases energy =
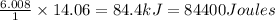
Thus 84400 Joules of energy is released when 253 g of liquid water freezes.