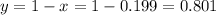Answer:
19.9% 10B and 80.1% 11B
Step-by-step explanation:
The average atomic mass of boron is calculated using the weighted average of the atomic masses of 10B and 11B.
A system of two equations can be written, where x represents the percentage (in decimal form) of 10B and where y represents the percentage (in decimal form) of 11B.
1.
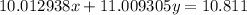
2.
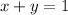 >>
>>
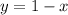
Inserting Equation 2 into Equation 2 gives:
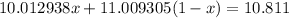
Solving for x gives:
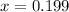
Substituting this value of x into Equation 2 above gives: