Answer:
(1)
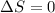
(2)
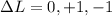 but
but
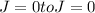 transition not allowed.
transition not allowed.
Step-by-step explanation:
Atoms can be described by the quantum number n, spin quantum number S, angular momentum quantum number L, and total angular momentum quantum number J. Based on approximation Russel- Saunders electron coupling, the atomic term symbol can be written as
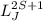 .
.
The conditions or selection rule to promoting the electron are discussed below:
(1) The total spin should not change that is
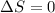 .
.
(2) The total angular momentum change should be,
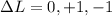 but
but
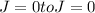 transition not allowed.
transition not allowed.