Answer:
Acetic acid and hydazine
Step-by-step explanation:
Hydrogen bonding is weak electrostatic force existing between a hydrogen atom covalently bonded to electronegative atoms and another elcetronegative atom.
Most commonly, compounds having hydrogen bonded to N, F and O atoms show hydrogen bonding.
Hydrogen bonding can be intermolecular and intramolecular.
Among the given option, acetic acid and hydrazine have hydrogen bonding.
Molecular formula of acetic acid is
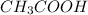 , so only acetic has hydrogen bonded to electronegative atom 'O'.
, so only acetic has hydrogen bonded to electronegative atom 'O'.
Molecular formula of acetone is
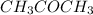 . It has no hydrogen bonded to electronegative atom.
. It has no hydrogen bonded to electronegative atom.
Molecular formula of hydrazine is
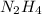 . In this molecule, hydrogen is bonded to electronegative atom 'N'.
. In this molecule, hydrogen is bonded to electronegative atom 'N'.