Answer: The equation is written below.
Step-by-step explanation:
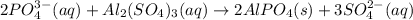
According to Stoichiometry of the reaction:
2 moles of phosphate ions reacts with 1 mole of aluminum sulfate to produce 2 moles of aluminum phosphate precipitate and 3 moles of sulfate ions.
Aluminum phosphate is an odorless and white crystalline solid
The chemical equation is written above.