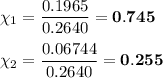Answer:
0.745 and 0.245
Step-by-step explanation:
Mole fraction (χ) is the number of moles of a component divided by the total number of moles in a mixture.
We must calculate the moles of each component.
Let CO₂ be Gas 1 and SO₂ be Gas 2.
1. Calculate the moles of each gas.
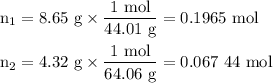
2. Calculate the total moles.
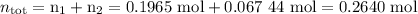
3. Calculate the mole fraction of each component