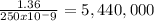Answer:
5,440,000 atoms
Step-by-step explanation:
The width (diameter) of a chromium atom is 250 pm (twice the radius). A picometer is equal to 10⁻⁹ millimeters, so the width of an atom is 250 x 10⁻⁹ mm
The number of atoms required to span of 1.36 mm is calculated as follows: