Answer:
-3,89
Step-by-step explanation:
First to consider is that the reaction is raising the temperature of the solution, so it's an exothermic reaction.
The enthalpy can be calculated by the following equation:
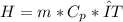
First we need to calculate the mass using the density and the volume:
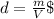 ⇒
⇒
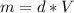
![m=250[mL]*1,25[(g)/(mL)]=312,5 [g]](https://img.qammunity.org/2020/formulas/physics/high-school/szv4b0tg0q0yqq8s0qqmskwgq28gf5w2y3.png)
Then we have Cp = 3,74 Joules/gram°K ΔT = -3,33 °C (cause of exothermic reaction)
Replacing in the formula:
![H=312,5*3,74*(-3,33)=-3891 [J]=-3,81[kJ]](https://img.qammunity.org/2020/formulas/physics/high-school/7oyggrhpwqlwk47s3kl6sgpbo0lzdy75if.png)