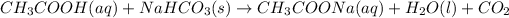Answer:
We test the materiel by adding small amount of it into a vinegar solution.
Step-by-step explanation:
Acids when reacts with sodium bicarbonate to give salt aqueous solution of salt , water and effervescence of the gas.
So, if we want to check whether our household product has sodium bicarbonate we will add small amount of house hold product to a small amount(3 -4 mL) of vinegar solution(10% acetic acid).
- If effervescence is observed then it confirms the presence of sodium bicarbonate.
- If no effervescence is observed then it confirms the absence of sodium bicarbonate.
The reaction between vinegar and sodium bicarbonate is given as: