Answer:
The percentage of error in this experiment is 0.66%
Explanation:
The value measured by a student, let's call it
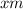 , is equal to 0.1355 M. And the real value of molarity is,let's call it
, is equal to 0.1355 M. And the real value of molarity is,let's call it
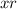 is equal to 0.1364.
is equal to 0.1364.
The error in the measurement (E) is equal to the absolute difference between the real value and the measured value:
abs ( xr - xm ) = E
So in this experiment it will be equal to:
abs ( 0.1364 - 0.1355 ) = 0.0009
And the percentage of error (e) will be:
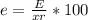
Thus,
e = 0.0009 * 100 / 0.1364 ⇒ e= 0.659 % ≈ 0.66 %
So, the percentage of error in this experiment is 0.66%