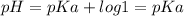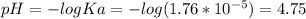Answer:
pH = 4.75
Step-by-step explanation:
Given:
Ka(acetic acid, CH3COOH) = 1.76*10^-5
Volume of CH3COOK = 50.0 ml
Molarity of CH3COOK = 1.00 M
Volume of CH3COOH = 50.0 ml
Molarity of CH3COOH = 1.00 M
To determine:
pH of the above buffer solution
Calculation:
The pH of a buffer is related to the concentrations of the conjugate base and acid via Henderson - Hasselbalch equation:
![pH = pKa + log([A-])/([HA])](https://img.qammunity.org/2020/formulas/chemistry/college/ksu985vnkc52edohzh6cbdiorawz6zclyf.png)
where pKa = -logKa
{A-] = conjugate base
[HA] = acid
For the CH3COOH/CH3COOK buffer:
![pH = pKa + log([CH3COOK])/([CH3COOH])](https://img.qammunity.org/2020/formulas/chemistry/college/3mpwmvcj2j0mcfb041tvkspcre32fnn00e.png)
here, [CH3COOK] = [CH3COOH], therefore,