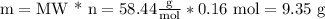Answer:
9.35g
Step-by-step explanation:
The molarity equation establishes that:
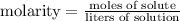
So, we have information about molarity (2M) and volume (80 ml=0.08 l), with that, we can find the moles of solute:
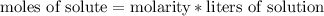
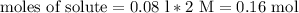
The mathematical equation that establishes the relationship between molar weight, mass and moles is:
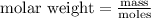
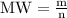
We have MW (58.44g/mole) and n (0.16 mol), and we need to find m (grams of salt needed) to solve the problem: