Answer:
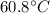
Explanation:
The heat absorbed
 is given by:
is given by:
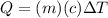 (1)
(1)
Where:
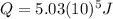
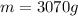 is the mass of water
is the mass of water
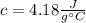 is the specific heat of water
is the specific heat of water
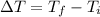 is the variation in temperature, being the final temperature
is the variation in temperature, being the final temperature
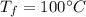 which is the boiling temperature of water
which is the boiling temperature of water
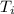 is the initial temperature
is the initial temperature
Finding
 :
:
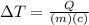 (2)
(2)
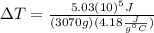 (3)
(3)
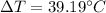 (4)
(4)
Knowing
 and
and
 we can find
we can find
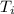 :
:
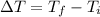
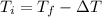 (5)
(5)
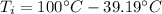 (6)
(6)
Finally:
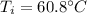 This is the initial temperature of the water
This is the initial temperature of the water