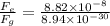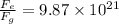Answer:
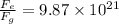
Step-by-step explanation:
As we know that the charge on electron and proton is same as the charge of an electron
so the electrostatic force between the electron and proton is given as
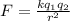
so we will have
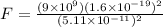
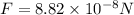
Now the weight of an electron is given as
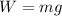
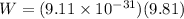
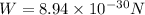
now the ratio of electrical force and weight of the electron is given as