Answer:0.09 M step by step in explanation
Step-by-step explanation:
First We need to considerated the equation of titrated of the KIO3
Then:
IO3 + 5I- + 6H+ ---> 3I2 + 2H2O
I2 + 2 S2O3 --> 2I- + S4O62-
Then We have that one mmol of IO3 produced 3 mmol of I2 and one mmol of I2 reacted with 2 mmol of S2O3( thiosulfate ion)
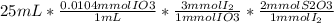
There is 1.56 mol S2O3
then we divide the result by 17.27mL to obtain the concentration of the thiosulphate solution
[Na2S2O3]=
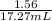
[Na2S2O3]=0.09M