Answer: 172.2 g of
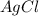 is formed by adding sufficient silver nitrate to react with 1500.0mL of 0.400M barium chloride solution.
is formed by adding sufficient silver nitrate to react with 1500.0mL of 0.400M barium chloride solution.
Step-by-step explanation:
To calculate the moles, we use the equation:
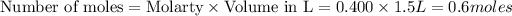
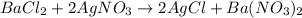
According to stoichiometry:
1 mole of
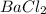 produce = 2 moles of
produce = 2 moles of
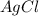
Thus 0.6 moles
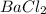 will produce =
will produce =
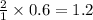 moles of
moles of
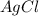
Mass of
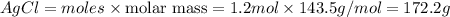
Thus 172.2 g of
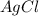 is formed by adding sufficient silver nitrate to react with 1500.0mL of 0.400M barium chloride solution.
is formed by adding sufficient silver nitrate to react with 1500.0mL of 0.400M barium chloride solution.