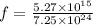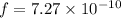Answer:
Total number of electrons
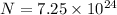
electrons removed from each sphere
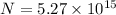
Fraction of electrons transferred is given as
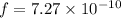
Step-by-step explanation:
As we know that moles is defined as
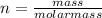
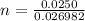
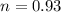
so number of atoms of Al in each sphere is given as
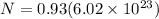
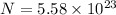
Now number of electrons in each atom is given as
atomic number = number of electrons in each atom = 13
total number of electrons in each sphere is
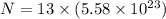
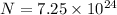
Also we know that force of attraction between them is given as
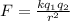
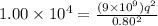
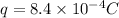
now we have
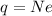
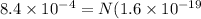
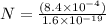
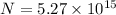
Fraction of electrons transferred is given as