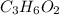Answer:
The empirical formula of this substance is:
Step-by-step explanation:
To find the empirical formula of this substance we need the molecular weight of the elements Carbon, Hydrogen and Oxygen, we can find this information in the periodic table:
- C: 12.01 g/mol
- H: 1.00 g/mol
- O: 15.99 g/mol
With the information in this exercise we can suppose in 100 g of the substance we have:
C: 48.64 g
H: 8.16 g
O: 43.2 g (100 g - 48.64g - 8.16g= 43.2 g)
Now, we need to divide these grams by the molecular weight:
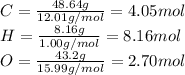
We need to divide these results by the minor result, in this case O=2.70 mol

We need to find integer numbers to find the empirical formula, for this reason we multiply by 2:
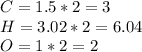
This numbers are very close to integer numbers, so we can find the empirical formula as subscripts in the chemical formula: