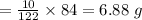Answer:
Minimum amount of
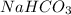 = 6.88 g
= 6.88 g
Step-by-step explanation:
Benzoic acid reacts with
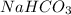 as:
as:
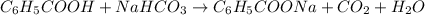
Molar mass of
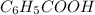 = 122 g/mol
= 122 g/mol
Molar mass of
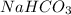 =84 g/mol
=84 g/mol
No. of moles of
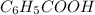 =
=
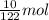
From reaction,
1 mol of
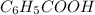 reacts with 1 mol of
reacts with 1 mol of
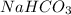
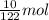 mol of
mol of
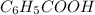 will react with
will react with
=
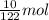 of
of
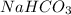
Mass of
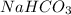 in g = No. of mol × Molar mass
in g = No. of mol × Molar mass
=