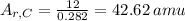Answer:
The relative mass of carbon would be
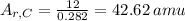
Step-by-step explanation:
The amu is defined as 1/12 of the mass of a
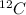 atom, thus according to the definition, the relative mass of carbon is exactly 12. If the definition of the AMU changed, we could calculate what the new relative mass is by dividing the relative mass of
atom, thus according to the definition, the relative mass of carbon is exactly 12. If the definition of the AMU changed, we could calculate what the new relative mass is by dividing the relative mass of
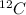 by the mass of the new AMU in old AMUs.
by the mass of the new AMU in old AMUs.
The atomic weight of Phosphorus is 30.973, therefore an amu would be
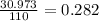 in old AMUs, if the atomic weight of carbon is 12.00, then in the new amu:
in old AMUs, if the atomic weight of carbon is 12.00, then in the new amu: