Answer:
The width L of the barrier is b. 0.116nm
Step-by-step explanation:
This is a case of tunnel effect when the probability is less than one.
First of all you find all the information the problem gives you:
Electron's total energy E=5eV
Height of potential barrier U=20eV
Crossing Probability T=0.03
Width of the barrier L=unknown
Then you need to find the equations to use in cases in which the probability is less than 0, so you have:
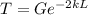
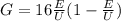
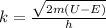 where m is the mass of the electron,
where m is the mass of the electron,
 , and h is the Planck constant,
, and h is the Planck constant,
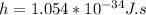
First we find G, we have:
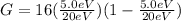
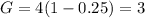
Now, to solving k, we need to find the difference (U-E) :
U - E = 20eV - 5eV = 15eV
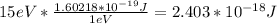
Then, we can find k:
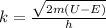

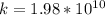
Finally, we solve for L the probability equation T:
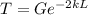
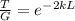
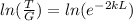
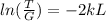
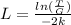
And replacing values for T, G and k, we can find the width of the barrier:
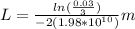
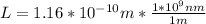
L=0.116nm