Answer: B. ΔH < 0, ΔS > 0, and ΔG < 0
Step-by-step explanation:
Exothermic reactions are those in which heat is released by the system and endothermic reactions are those in which heat is absorbed by the system.
 for Exothermic reaction is negative and
for Exothermic reaction is negative and
 for Endothermic reaction is positive.
for Endothermic reaction is positive.
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
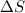 is positive when randomness increases and
is positive when randomness increases and
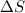 is negative when randomness decreases.
is negative when randomness decreases.
When NaOH is dissolved into water, the molecules change their state from solid to aqueous and thus randomness increases. The beaker becomes warm means the reaction is exothermic
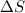 is positive and
is positive and
 is negative Using Gibbs Helmholtz equation:
is negative Using Gibbs Helmholtz equation:
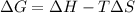
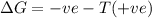
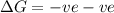
Thus
 will be negative.
will be negative.
Thus
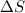 is positive,
is positive,
 is negative and
is negative and
 will be negative.
will be negative.