Answer: The correct answer is 20.2 g silver placed in 21.6 mL of water and 20.2 g copper placed in 21.6 mL of water
Step-by-step explanation:
Density of a substance is defined as the ratio of its mass and volume. The chemical equation representing density of a substance is:
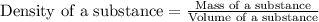
Density is considered as an intensive property. Intensive property is the property that does not depend on the amount of substance. It remains same.
If the masses and volume of different substances are same, they will have same density.
For 20.2 g silver placed in 21.6 mL of water and 20.2 g copper placed in 21.6 mL of water:
Density of silver =
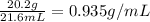
Density of copper =
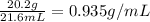
Hence, the correct answer is 20.2 g silver placed in 21.6 mL of water and 20.2 g copper placed in 21.6 mL of water