Answer: The correct answer is Option A and option D.
Step-by-step explanation:
Density of a substance is defined as the ratio of its mass and volume. The chemical equation representing density of a substance is:
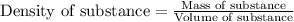
Density is an intensive property. Intensive property is defined as the property which does not depend upon the amount of substance. It remains same.
The density of a particular substance remains the same.
For Example: Density of iron will remain the same for 50 grams or 0.1 grams of iron sample which is 7.874 g/mL
Hence, the correct answer is Option A and option D.