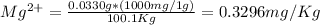Answer:
 = 4.0959 ppm
= 4.0959 ppm
 = 0.3296 ppm
= 0.3296 ppm
Step-by-step explanation:
ppm means milligram of solute per kilogram of solution.
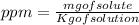
Solutes:
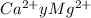
Mass
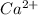 = 0.410g
= 0.410g
Mass
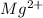 = 0.0330g
= 0.0330g
Solution: 100.0 L, whose density is 1.001 g/mL
First, let us to calculate mass of solution:
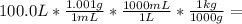 = 100.1 kg
= 100.1 kg
Now, we have to calculate ppm
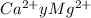
ppm
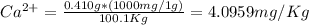
ppm