Answer:
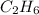
Step-by-step explanation:
The boiling point depends on the intermolecular forces between the molecules of the compound.
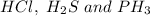 have dipole- dipole interactions because there is the difference in electronegativity values of the atoms in the molecules.
have dipole- dipole interactions because there is the difference in electronegativity values of the atoms in the molecules.
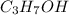 posses hydrogen bonding.
posses hydrogen bonding.
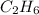 : The bond in the molecule is C-H, which is non-polar in nature because the carbon and the hydrogen having very similar electronegativity values.
: The bond in the molecule is C-H, which is non-polar in nature because the carbon and the hydrogen having very similar electronegativity values.
The intermolecular force acting in the molecule are induced the dipole-dipole forces or London Dispersion forces / van der Waals forces which are the weakest intermolecular force.
Hence, it has the weakest bond, so it takes least amount of the energy and hence the lowest boiling point.