Answer: The student’s values are accurate as well as precise.
Step-by-step explanation:
Precision refers to the closeness of two or more measurements to each other.
For Example: If you weigh a given substance three times and you get same value each time. Then the measurement is very precise.
Accuracy refers to the closeness of a measured value to a standard or known value.
For Example: If the mass of a substance is 50 kg and one person weighed 49 kg and another person weighed 48 kg. Then, the weight measured by first person is more accurate.
Given: Mass = 5.000 g
Mass weighed by A has values 4.891 g , 4.901 g and 4.890. Thus the average value is
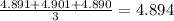
Thus as the measured value is close to the true value, the student’s values are accurate and as the values are close to each other, the measurement is precise.