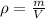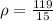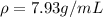Answer:
Step-by-step explanation:
Initial level of water in the cylinder is given as
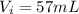
final level of volume in the cylinder is given as
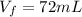
so volume displaced by the iron piece is given as
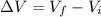
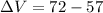
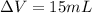
so by Archemedies principle the volume displaced by the object is always equal to the volume of the object itself
so here volume of object is 15 mL
mass of the iron piece is 119 g
so here the density of the iron piece is given as