Answer:
13.54 kJ
Step-by-step explanation:
First, it is necessary to know the chemical reaction.
Cr(s) +
 (g) →
(g) →
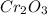
This reaction is not balanced, the balanced reaction is:
4Cr(s) + 3
 (g) →2
(g) →2
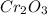
With this in mind, now have to find the moles of Cr for this it is necessary to divided 1.25 g of Cr by the molecular weight 51.99 g/mol. The result is 0.024 mol of Cr. Now using the balance equation, 4 moles of Cr will produce 2 moles of
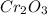 . Then 0.024 mol of Cr → 0.012 mol of
. Then 0.024 mol of Cr → 0.012 mol of
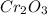 .
.
Now it is necessary to use the Std. enthalpy of formation value for the
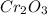 (-1128 kJ/mol). This is the amount of heat produce when one mol is produce. So now is just multiple this value by the amount of moles produce (0.012). Founding that 13.54 kJ of energy are produced when 1.25 g of Cr react will oxygen under Std. conditions.
(-1128 kJ/mol). This is the amount of heat produce when one mol is produce. So now is just multiple this value by the amount of moles produce (0.012). Founding that 13.54 kJ of energy are produced when 1.25 g of Cr react will oxygen under Std. conditions.