Answer: The
 and
and
 of the reaction is
of the reaction is
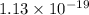 and -108080 J respectively.
and -108080 J respectively.
Step-by-step explanation:
For the given cell reaction:
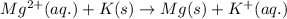
The half reaction follows:
Oxidation half reaction:
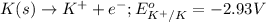
Reduction half reaction:
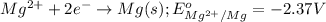
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
To calculate the
 of the reaction, we use the equation:
of the reaction, we use the equation:
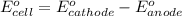
Putting values in above equation, we get:
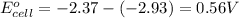
- To calculate Gibbs free energy, we use the equation:
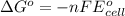
where,
 = Standard Gibbs free energy of the reaction = ?
= Standard Gibbs free energy of the reaction = ?
n = number of electrons exchanged = 2
F = Faraday's constant = 96500
 = standard electrode potential of the cell = 0.56 V
= standard electrode potential of the cell = 0.56 V
Putting values in above equation, we get:
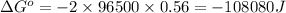
- To calculate the equilibrium constant of the reaction, we use the equation:
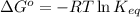
R = Gas constant = 8.314 J/K.mol
T = temperature of the reaction =
![25^oC=[273+25]=298K](https://img.qammunity.org/2020/formulas/chemistry/college/6emvaajqo5qvucrhq2qn2dbo2gul9o60b4.png)
 = equilibrium constant of the reaction = ?
= equilibrium constant of the reaction = ?
Putting values in above equation, we get:
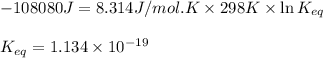
Hence, the
 and
and
 of the reaction is
of the reaction is
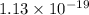 and -108080 J respectively.
and -108080 J respectively.