Answer: The solubility equilibrium equation is written below.
Step-by-step explanation:
Solubility is defined as the maximum amount of solute that can be dissolved in a solvent at equilibrium.
Solubility product is defined as the product of concentration of ions present in a solution each raised to the power its stoichiometric ratio. It is represented as

Calcium lactate is an ionic compound formed by the combination of 1 calcium ion and 2 lactate ions.
The equilibrium reaction for the ionization of calcium lactate follows the equation:
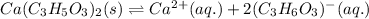
The solubility product for the above reaction is:
![K_(sp)=[Ca^(2+)]* [C_3H_6O_3^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/ftktnhb64bc6dshxab9u1fmlf6vuo3c560.png)
Hence, the solubility equilibrium equation is written above.