Answer:
The final temperature of water is 381.39 °C.
Step-by-step explanation:
Given that
Mass of water = 5 kg
Heat transfer at constant pressure Q = 2960 KJ
Initial temperature = 240 °C
We know that heat transfer at constant pressure given as follows
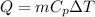
We know that for water
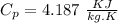
Lets take final temperature of water is T
So
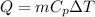
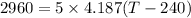
T=381.39 °C
So the final temperature of water is 381.39 °C.