Answer:
7.92 g/ml
Step-by-step explanation:
Given that, the volume of water in the graduated cylinder is,
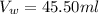
Volume of (water+iron shot) is,
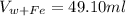
Now the volume of iron shot can be calculate as,

And the mass of the iron shot which is given is 28.5 g.
Now the density can be calculated as,
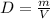
Here, m is the mass, V is the volume
Now,
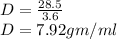
Therefore, the density of iron is 7.92 g/ml.